Your partner for high-purity water solutions—from concept to commissioning. Turnkey delivery with compliance built in.
Our Services
Pretreatment Systems
Customized pretreatment systems engineered to deliver consistent potable-quality water, safeguarding downstream Purified Water systems. Enhances operational efficiency, reliability, and long-term system performance.
PW Generation (RO+EDI)
High-performance RO–EDI Purified Water systems designed for pharmaceutical applications with hot and chemical sanitization. Integrated automation ensures reliable, compliant, and consistent performance.
WFI Generation
Membrane-based WFI generation systems built with a multi-barrier approach for pharmacopeia-compliant water quality. Designed to reduce energy consumption while lowering overall carbon footprint.
Storage & Distribution
SS 316L electropolished tanks and distribution loops with dead-leg–free design, orbital welding, and high-purity piping. Engineered for maximum integrity, effective sanitization, and complete drainability.
Ozonation System
Advanced ozone-based solutions designed to eliminate pathogenic bacteria and ensure effective water disinfection. Optimized application strategy delivers consistent, reliable, and safe performance.
Lifecycle Services
Comprehensive lifecycle support including preventive maintenance, validation, and expert troubleshooting. Ensures dependable system performance with minimized downtime and lower operating costs.
Compliance-First Engineering
Compliance-Ready Engineering. Audit-Ready Execution.
We don’t just supply systems—we deliver complete compliance solutions that protect patient safety and your reputation.
GMP/WHO standards integrated from design phase with comprehensive validation support and documentation
Advanced PLC/SCADA systems with real-time monitoring, automated logging, and alarm management
Complete documentation packages including DQ/IQ/OQ protocols, SOPs, and maintenance records
End-to-end ownership from concept to commissioning with dedicated post-installation support
- GMP / WHO
- 21 CFR Part 11
Optional Ready
- PLC/SCADA
- IQ/OQ/PQ
- 100%
- Zero
- 24x7
Industry-leading systems engineered for pharmaceutical excellence

- PW Generation System (RO + EDI)
Advanced pharmaceutical-grade purified water with multi-stage purification
- Robust RO Design – Single/Dual-pass configuration for consistent dissolved solids reduction
- EDI Polishing – Continuous deionization without chemical regeneration
- Sanitary & GMP-Compliant – SS 316L contact parts, orbital welding, hygienic fittings
- Hot Water or Chemical Sanitizable – Effective sanitization protocols for microbiological control
- Advanced PLC-Based Automation – Recipe-based operation, alarms, data logging, audit-ready
- Validated & Documented – Comprehensive DQ, IQ, OQ support, SOPs included
- Cold WFI Generation System
Energy-efficient alternative to traditional distillation
- Membrane-Based WFI – Advanced non-thermal purification technology
- Pharmacopeia-Aligned Quality – Meets USP, EP, WHO standards
- Multi-Barrier Technology – RO, EDI, and Post UF for endotoxin control
- Advanced Automation – PLC-based control with 21 CFR Part 11 option
- Lower Operating Costs – Reduced utilities and chemical-free operation
- Validation Support – Comprehensive DQ, IQ, OQ documentation
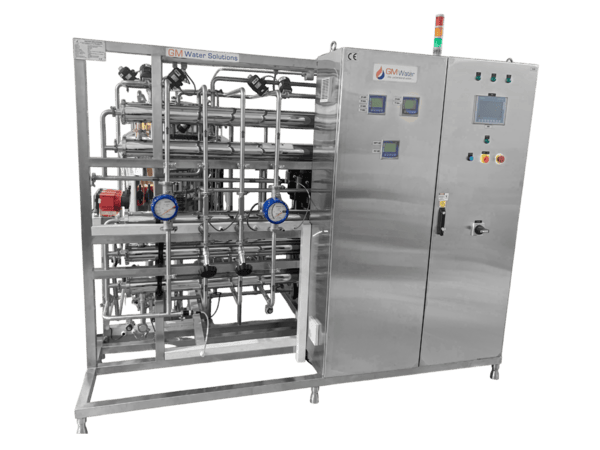

- Storage & Distribution System (PW / WFI)
Hygienic continuous re-circulation for pharmaceutical water systems
- Hygienic Continuous Re-circulation – Prevents stagnation and supports microbiological control
- GMP-Compliant SS 316L Construction – Orbital welding and sanitary fittings
- Velocity-Based Recirculation – Turbulent flow for consistent water quality
- PLC-Based Automation – Real-time conductivity, TOC, temperature monitoring
- Hot Water, Ozone & Pure Steam Sanitizable – Validated sanitization strategies
- 21 CFR Part 11–Ready – Optional data integrity for validated environments
- Ozonation System
Effective control of pathogenic bacteria using Air-Based Corona Discharge Technology
- Designed for Pharmaceutical Water plants to effectively control bacteria, biofilm, and organic contamination without chemicals.
- Residue-Free Disinfection Ozone naturally decomposes to oxygen – no harmful chemical residues.
- High Mass Transfer Efficiency Optimized venturi injector/static mixer for maximum ozone dissolution.
- Real-time measurement for precise dosing and process control using Online Ozone Analyzer
- Ozone Destruct Unit for Off-Gas Safety Catalytic destruction system ensures safe working environment.
- Validation Support (DQ/IQ/OQ Documentation) Comprehensive documentation package for regulatory compliance.
- Low Operating Cost No chemical storage, reduced downtime, minimal maintenance.
- Compact & Modular Skid Design Easy integration with new or existing water treatment systems.

Why Choose GM Water?
Proven expertise, comprehensive solutions, and unwavering commitment to your success
- Deep Industry Expertise
20+ years of pharmaceutical water systems experience with proven track record
- Proven Reliability
Consistent performance with minimal downtime and maximum uptime
- End-to-End Turnkey Delivery
From concept to commissioning, single-point accountability throughout
- Strong Support Network
24×7 service availability with rapid response and qualified technicians
- Compliance-Ready Systems
Built-in GMP/WHO standards with validation support and audit readiness
- Sustainability Focus
High recovery rates, energy efficiency, and environmental responsibility
Ready to discuss your water system requirements?
Comprehensive service programs to maximize uptime, ensure compliance, and extend system life
AMC / Preventive Maintenance
Scheduled maintenance programs to prevent breakdowns, ensure compliance, and extend equipment life with 24x7 support availability
Reduced downtime
Compliance assurance
Cost predictability
Design Consultation
Qualified personnel managing your water systems operations, reducing risks and ensuring optimal performance throughout the asset lifecycle
Expert operation
Reduced operational risk
Extended asset life
Plant Upgradation
System improvements including recovery optimization, TOC integration, loop expansion, and 21 CFR Part 11 compliance upgrades
Enhanced efficiency
Improved compliance
Future-ready systems
Keep Your Water Systems Running at Peak Performance
Our service programs are designed to prevent issues before they occur, ensuring continuous compliance and optimal operation.
Specialized water systems for diverse pharmaceutical and healthcare applications
Pharmaceutical Manufacturing
Sterile/Injectables
API Units
Biotechnology
Research Labs
Delivering tailored solutions that meet the unique requirements of each industry segment
Real outcomes from pharmaceutical facilities we’ve partnered with
- 100% Paperless Record
- Compliance Transformation
Leading Injectable Manufacturer
Challenge:
Logsheet maintenance for Multiple PW/WFI Storage & Distribution systems was time consuming and person dependent.
Solution:
Replaced 13 numbers of control panels for different PW/WFI systems and Implemented 21CFRpart 11 with minimum downtime.
Outcome:
Save time of plant operators by implementing 100% paperless record.
- 75% Recovery
- Recovery Optimization
API Manufacturing Facility
Challenge:
Low RO recovery rate (45%) leading to high operational costs and water wastage
Solution:
Upgraded pretreatment and RO system with advanced membrane technology
Outcome:
Increased recovery to 75%, saving 2.5M liters annually with 40% cost reduction
- 99.8% Uptime
- Zero Downtime Achievement
Multi-Site Pharma Company
Challenge:
Frequent system breakdowns causing production delays and compliance risks
Solution:
Implemented comprehensive AMC program with predictive maintenance protocols
Outcome:
Achieved 99.8% uptime over 24 months with complete breakdown elimination
Watch demonstrations of our pharmaceutical water systems delivering consistent, compliant results

PW Generation System
Complete PW generation system with RO+EDI technology

PW Distribution Skid
Purified water storage and distribution loop in operation

WFI Distribution Skid
Water for injection distribution system demonstration
Want to see a personalized demonstration of our systems?
Expert insights on pharmaceutical water systems, compliance, and best practices
Preventive Maintenance: The Key to Water System Reliability
When to Consider Water System Upgradation
Common Water Quality Issues and Solutions